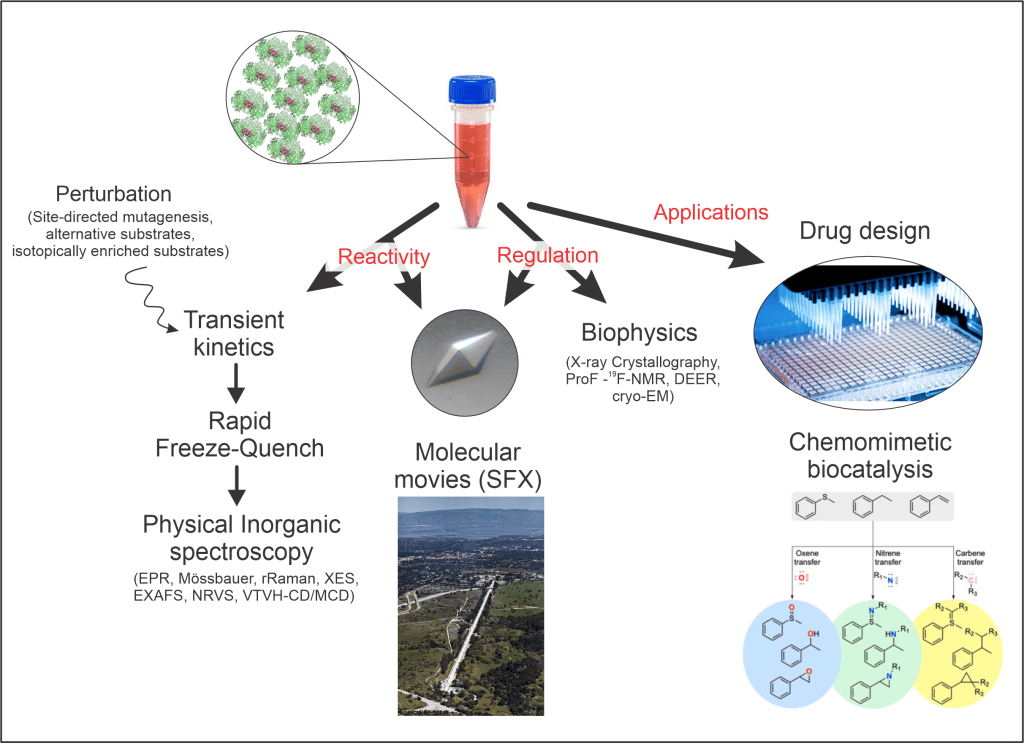
Oxygen activation and the regulation of the powerful reactive oxygen species that result from it are fundamental biological processes common to all aerobic organisms. Oxygenase enzymes of many types have been identified that activate O2 and insert oxygen atoms into biological molecules, while at the same time preventing oxidative damage to themselves. To better understand the mechanisms of these dual aspects of catalysis, we will study two members of the dinuclear iron oxygenase family that appear to follow distinct tracks to achieve the common goal of functionalizing unactivated C-H bonds. A unified study of soluble methane monooxygenase (sMMO) and a member of the integral membrane desaturase (IMD) family [stearoyl-CoA desaturase] will allow us to define the distinct roles of the metal center, its ligands, the protein, and the membrane environment in the biological chemistry of O2 utilization.
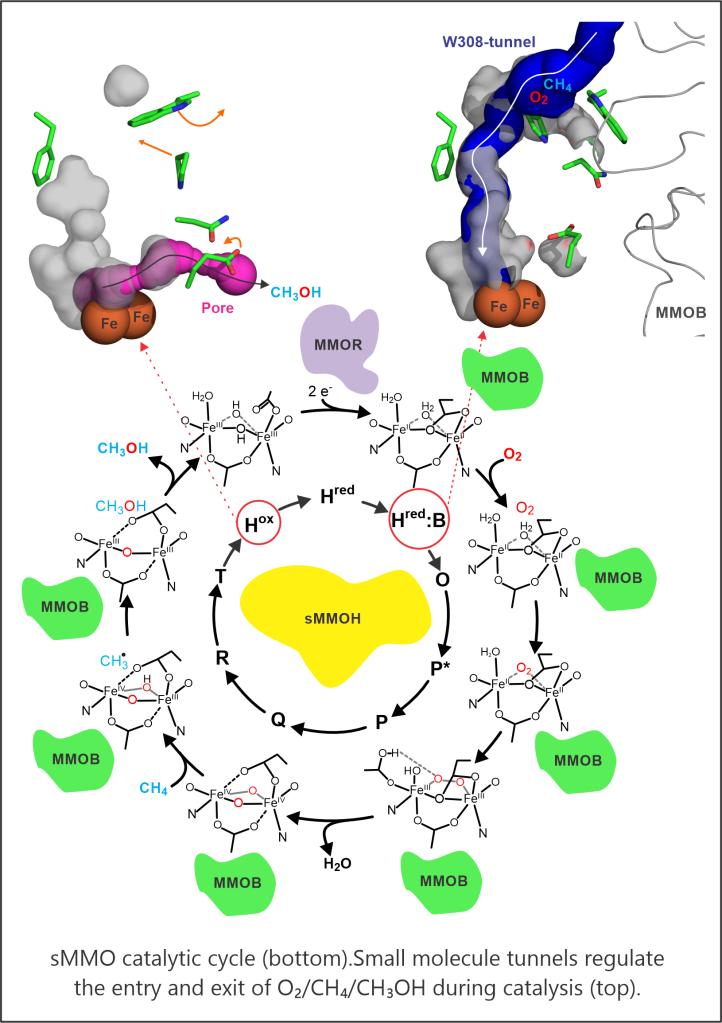
These two facets of catalysis, namely chemical reactivity and its regulation, are aptly reflected in sMMO, which catalyzes the O2-dependent conversion of methane to methanol in the C1 metabolism of methanotrophs. The smallest hydrocarbon with the strongest aliphatic C-H bond is selectively oxidized amidst a sea of easier-to-oxidize alternative substrates and under reaction conditions green enough to shame industrial Fischer-Tropsch synthesis. Because sMMO generates Nature’s most powerful oxidant, an oxygen-bridged dinuclear iron(IV) species termed Q, for cleaving the C-H bond of methane, this enzyme will oxidize any organic molecule with a C-H or C=C bond that can access the active site. An understanding of how sMMO selectively oxidizes methane most directly informs the design of biomimetic synthetic catalysts for strong C-H bond functionalization reactions and to revolutionize the use of natural gas as a liquid fuel and chemical industry feedstock. In addition, once such catalysts have been devised, they also need to be regulated to ensure productive turnover and we can draw inspiration from how the oxygenases manage this difficult task. Our study of sMMO has taught us that in as much the reactive iron-oxygen intermediates capture the limelight, it is the mechanisms of regulation that truly showcase the catalytic prowess of enzymes. For example, a small molecule tunnel in sMMO that delivers the right substrate at the right place and at the right time is emblematic of how these tunnels act as extensions of the active site in many metalloenzymes.
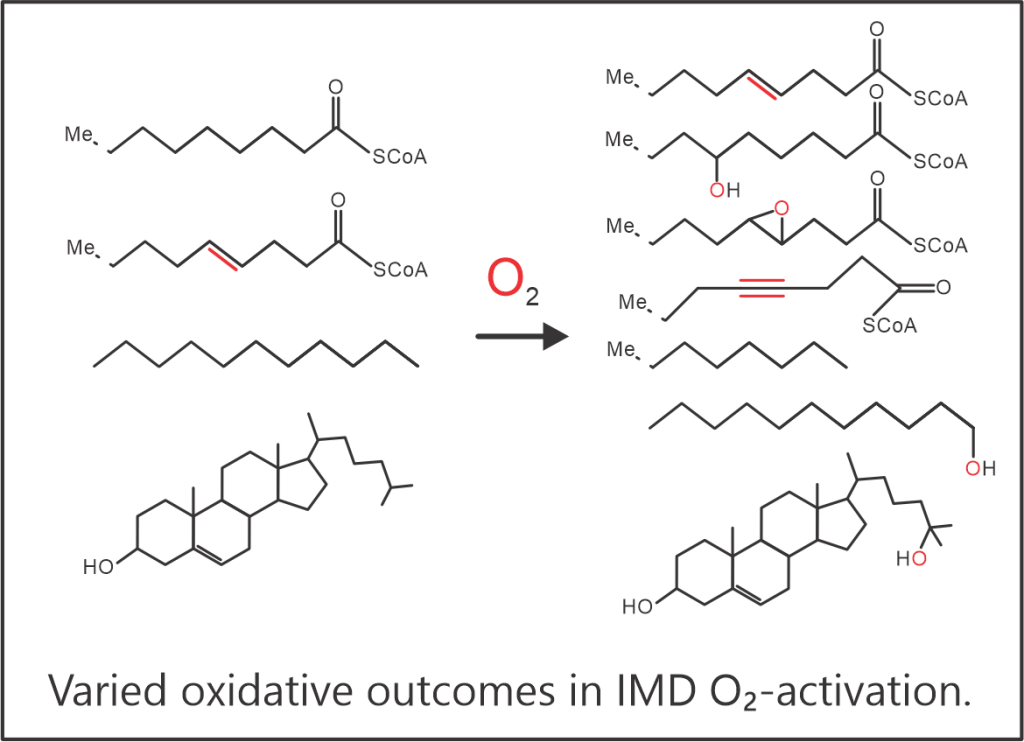
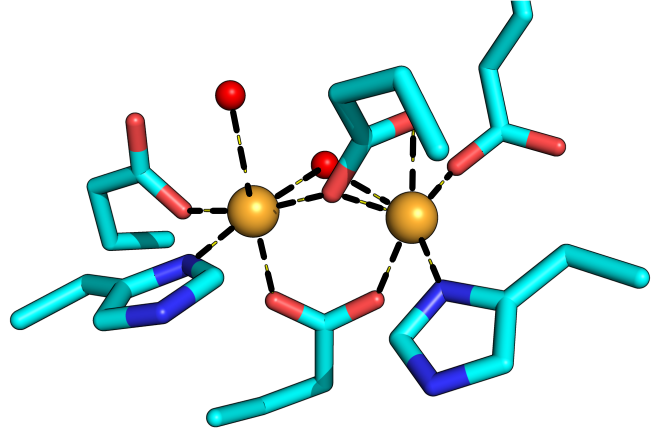
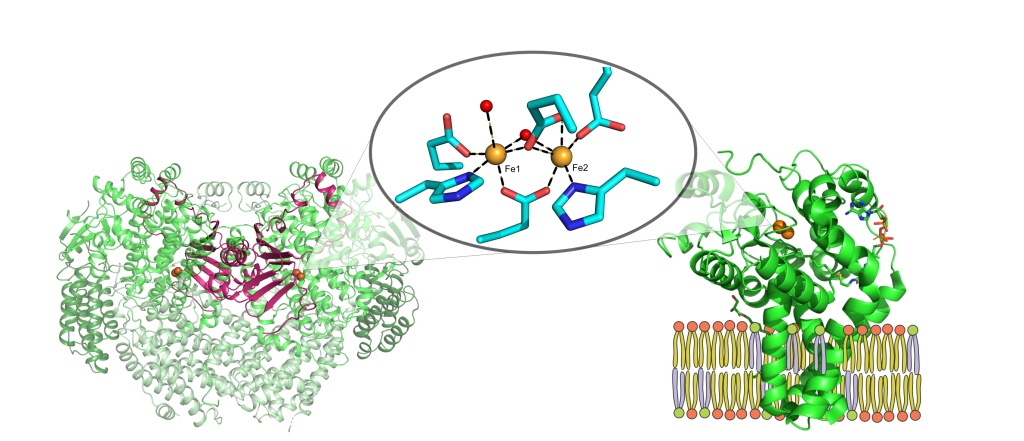
The ability of oxygenases to choose between different oxidative outcomes, be it desaturation, hydroxylation or decarboxylation, while also managing regiospecific conversions, is illustrated by the IMD family of enzymes. These enzymes activate O2 to catalyze primarily the desaturation and hydroxylation of sterols and fatty acids linked to various headgroups. The reaction products are critical to mammalian cell metabolism with the unsaturated fatty acids influencing membrane fluidity (mono- and poly-unsaturated fatty acids) and cell signaling (plasmalogen, sphingolipids) and the oxysterols (25-hydroxycholesterol) modulating the immune response. Stearoyl-CoA desaturase (SCD) catalyzes the desaturation of coenzyme-A linked stearic acid to oleic acid and is the prime candidate for study because it serves as the major non-dietary source of unsaturated fatty acids in humans. Our lab aims to investigate the potentially novel mechanism for O2-activation in SCD as inferred from the unique nitrogen-rich, 8-histidine diiron active site. The central role of SCD in generating membranes means that SCD expression levels are upregulated in cancer cells and during (+) RNA virus infections for making host-membrane derived replication complexes. Our lab, therefore, also aims to guide a rational in-vitro design of SCD inhibitors as anti-cancer and novel anti-viral therapeutics.